Clinical Applications
Cancer
In 1995,professor Law patented the use of myoblasts in treating cancer.
An article entitled of “Myoblasts Inhibit Prostate Cancer Growth by Paracrine Secretion of Tumor Necrosis Factor-α”was published in THE JOURNAL OF UROLOGY in may 2013.Both in vivo and in vivo experiments confirments that myoblasts inhibit prostate cancer growth and limit metastasis formation by paracrine tumor necrosis factor-α secretion.
The validity of the myoblast therapy in cancer has been further tested and verified.

Figure 1. Co-culture effects on myoblasts and cancer cells. Cell growth rate, differentiation ratio, morphology and gene expression were influenced by co-culture. Prostate carcinoma and sarcoma cell growth significantly decreased (a, b and e) and cells underwent apoptosis and/or cell cycle arrest (b and g). Mb, myoblasts (e, f and h). Apoptotic pathway activation was determined by staining for cleaved caspase 3 (Asp175). Positive staining was found in DU145 cells in co-culture with myoblasts (a) but not in tumor control tumor(b). Desmin staining in co-culture (c) and control (d) showed evident differentiation of myoblasts into well organized myotubes. Cytoskeleton was labeled with phalloidin 488 (green areas). Secondary antibody was labeled with Cy3 (red areas). Caspase 3 and p21 mRNA fold increase and protein expression significantly increased vs tumor control (g). mRNA fold increase was normalized to 18S reference gene. Dashed line indicates 1.0. Myoblasts differentiated rapidly in presence of tumor, significantly increasing differentiation ratio and decreasing cell growth (f and h). cont, control. +Mb, co-culture samples with myoblasts. -Mb, controls without myoblasts. Single asterisk indicates p<0.05 (h). Double asterisks indicate p <0.001 (e to h).
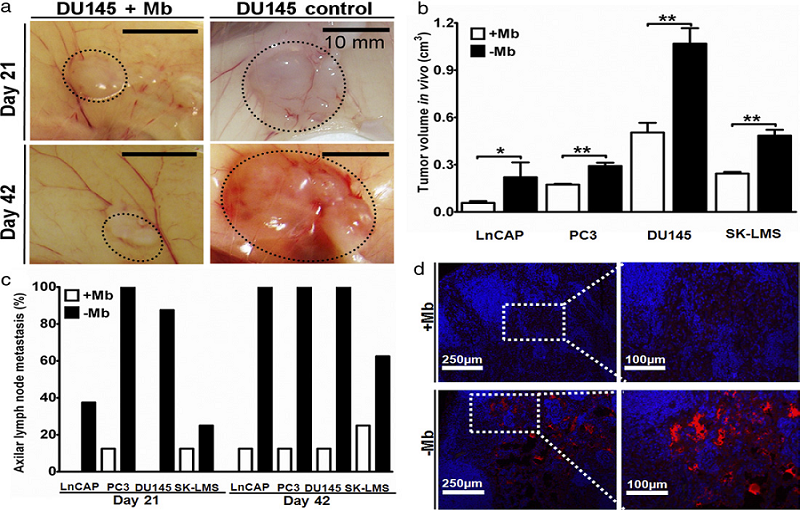
Figure 2. The results of myoblasts inhibit prostate cancer growth in vivo
Myoblasts and tumor cells were cultured and mixed with a collagen carrier (1 mg/ml) and bilaterally injected into the dorsal subcutaneous space of 8 nude mice per group (16 samples). Cell-cell interactions in vivo were examined on days 21 and 42 after injection using 9groups, including myoblasts alone (5x106), myoblasts co-injected with each of the 4 cancer cell lines (LNCaP, PC3, DU145 and SK-LMS, 2.5x106) and the 4 tumors alone (2.5x106).
Tumor growth and lymph node metastasis were reduced in vivo in samples co-injected with myoblasts. Tumors were measured 21 days after subcutaneous cell injection (a). Significant tumor size difference was found between co-injected and control samples. On day 42 myoblast co-injected tumor mass shrank but control samples continued to grow. Final tumor size at day 42 was significantly smaller in myoblast co-injected samples (b). Axillary lymph node metastasis assessment was performed by analyzing metastases (c). Ln ratio of axillar metastasis was significantly decreased in all tested cancers co-injected with myoblasts . H&E, desmin and cytokeratin staining for positive lymph nodes. Mice axillary lymph node 3 weeks after subcutaneous injection of LNCaP with and without myoblasts (d).